In the latest issue of Molecular Cell, Prof. Carina Baer de Oliveira Mann (Professor of Biomolecular Cryo-Electron Microscopy) and her team, in collaboration with clinicians and virologists, uncover the molecular basis of OAS2 function during viral infection with positive strand RNA viruses, such as COVID-19. Importantly, their work shows how mutations of OAS2 are implicated in autoimmune disease, thus providing a new way to understand and approach autoimmune diseases.
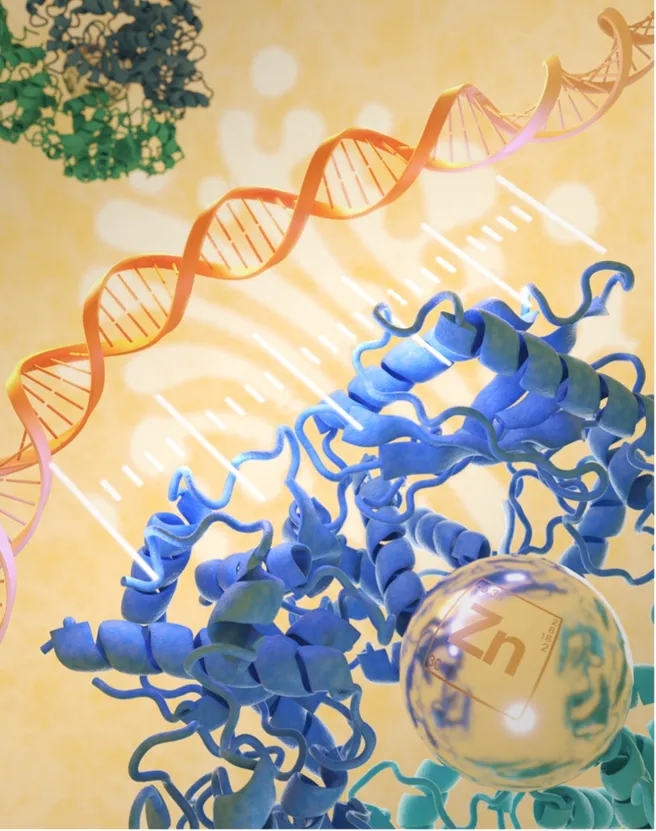
Genetic material can be stored and passed on in one of two ways: as RNA as in certain viruses or as DNA, as in plants and mammals. That said, RNA has an important role in plants and mammals too, serving amongst others as an intermediate to allow the translation of genetic material into proteins. Oligoadenylate synthetase 2(OAS2) is an immune receptor that senses viral double-stranded RNA in the cell leading to RNA degradation via RNase L. The level of OAS2 in cells is increased by the interferon pathway, one of several responses of our immune system upon viral infection. This could potentially create a problem if OAS2 would bind the cell’s own (i.e., endogenous) RNAs. So how does the cell ensure that only viral RNA is recognized?
Detailed analysis by Mann and coauthors reveals that OAS2 is exclusively located at the Golgi membrane on the outside of this structure facing the cytosol of the cells. This localization is crucial for its function and is mediated both by modification of the protein through myristoylation (a so-called post-translational modification), and by zinc-coordinated dimerization of two OAS2 molecules. Importantly, dimeric OAS2 is inactive. Activation of OAS2 is achieved through binding of (viral) RNA, whereby a specific domain of the OAS2 molecule acts as a ruler “measuring” the length of the bound RNA, thus avoiding autoreactivity with the cell’s own RNA molecules. Moreover, the membrane localization of OAS2 allows it to recognize viral RNA from such viruses as Corona viruses that exploit the endomembrane system of the host cell for replication, that is, viruses that try to “hide” the replicating viral RNA within double-membrane vesicle structures obtained from the host organelles.
The importance of this tightly controlled mechanism of OAS2 function is illustrated by clinical studies. DNA analysis of children with either early onset immune dysregulation or with Multisystem Inflammatory Syndrome (MIS-C) revealed OAS2 mutations in the catalytically active domain as well as in residues mediating RNA binding and protein stability, confirming the biological significance of these functions and indeed of the OAS2 protein itself. These findings have important medical implications in autoimmune disease and in response to viral infections, however, screening for OAS2 mutations is not (yet) part of clinical protocols.
Publication
Merold, Bekere, Mann, et al. Molecular Cell. doi.org/10.1016/j.molcel.2025.05.001
Further informations and Links
- Prof. Carina Baer de Oliveira Mann, Professorship of Biomolecular Cryo-Electron Microscopy
- Alumni doing research: TUM Professor Carina Baer de Oliveira Mann “I think to myself every day: I have no regrets” https://www.community.tum.de/en/carina-baer-de-oliveira-mann/
Contact to the article
Prof. Carina Baer de Oliveira Mann
Assistant Professorship of Biomolecular Cryo-Electron Microscopy
carina.mann(at)tum.de
https://www.bio.nat.tum.de/en/cryoem/home/
Press contact