The consortium is coordinated by Prof. Angela Casini, Chair of Medicinal and Bioinorganic Chemistry at the Department of Chemistry, and comprises five European teams that bring together complementary skills, technologies, and expertise in synthetic chemistry, radiopharmaceutical biology, as well as cancer diagnosis and therapy. Recent experiments from TUM and collaboration partners led by Jason Holland, Professor of Medicinal Radiochemistry at the University of Zurich (UZH), Switzerland, have shown that novel therapies can be made using self-assembly. In parallel, the consortium team headed by Jordi Llop radiochemistry and nuclear imaging expert at the Center for Cooperative Research in Biomaterials (CICbiomaGUNE) in San Sebastian, Spain, have shown efficient tumor therapy using radioactive nanorobots that use chemical fuels to find their targets faster.
SMARTdrugs will combine these ideas and explore how the new drugs can improve the therapy of lung cancers and brain tumors, conducted by the team of Dr. Tim Witney, molecular imaging specialist at King’s College London, UK, and by the group of Dr. Alex Poot, expert in radiology and nuclear medicine from Utrecht at the Princess Maxima Hospital and the University Medical Center in Utrecht, The Netherlands, respectively.
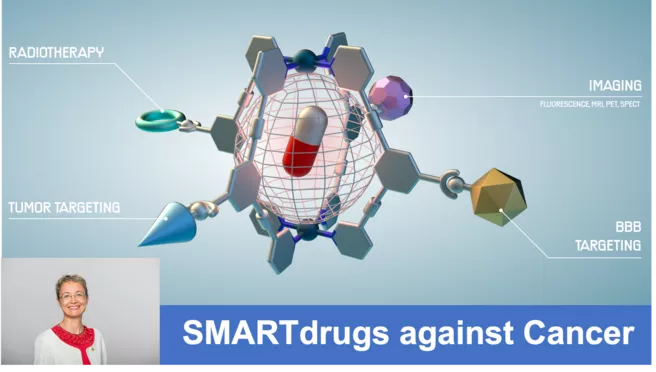
Now “SMARTdrugs” has been funded as a Pathfinder Open Grant by the European Innovation Council (EIC) with nearly 4 million Euros. “Our aim is to develop a new class of multifunctional supramolecules combining different radionuclides to image and treat two aggressive cancer types that currently have very poor prognosis for the patients affected. ” says Angela Casini.
Radionuclides are used for cancer diagnostic and therapy
For oncologists it’s important to recognize the size and location of cancerous tumors, for example in the lungs or brain, to choose the best therapy option for an individual and to follow the treatment over time to see if it works. Tumors can be made visible by using so-called radiotracers: a radionuclide, a radioactive isotope of an element, linked to a molecule which recognizes the cancer cells with high accuracy. Such radioactive drug molecules allow clinicians to see key signatures of tumors using state-of-the-art camera systems. The radioactive component produces light which can be detected by special imaging techniques like positron emission tomography (PET) providing highly accurate measurements.
Radionuclides can also be used to treat certain tumors. Again, the radionuclide is coupled to a drug molecule which guides the small radioactive payload in a targeted manner to the desired location. Specific accumulation of the therapeutic radiotracer in a tumor kills the cancer cells while sparing the surrounding healthy tissue. Instead of linking radionuclides directly to drug molecules, the researchers will create so-called ‘supramolecular compounds’ with improved control over size, shape, and other biochemical features that determine how well the new compounds perform in human tissue.
Better therapies for non-small cell lung cancer and pediatric brain tumors
SMARTdrugs will focus on non-small cell lung cancer in adults and brain cancers in children – aggressive cancer types of substantial unmet need. The 5-year survival rates are only 15% and 5%, respectively, and medicine has struggled to improve over the last 10 years despite advances in prevention, screening, and treatment. Lung cancer is the most common cause of cancer death world-wide and is classified into distinct histological subtypes, with non-small cell lung cancer accounting for approximately 85% of cases. Paediatric brain cancers contain multiple subtypes, like medulloblastoma or diffuse midline glioma, some of which have a life expectancy of less than a year after diagnosis. Current treatments often fail due to the mutations that lead to therapy-resistant tumor cells.
Contact: Prof. Dr. Angela Casini
School of Natural Sciences
Department of Chemistry
Technical University of Munich
E-mail: angela.casini@tum.de
Website: https://smartdrugs2024.com/