The transition to renewable energy requires efficient methods for storing large amounts of electricity. Researchers at the Technical University of Munich (TUM) have developed a new method that could extend the lifespan of aqueous zinc-ion batteries by several orders of magnitude. Instead of lasting just a few thousand cycles, they could now endure several hundred thousand charge and discharge cycles.
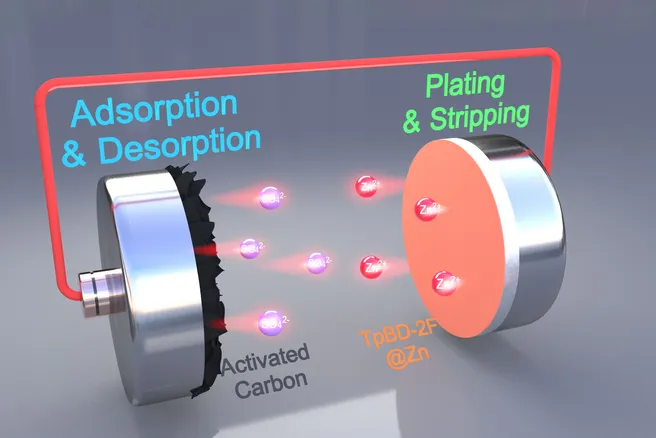
The key to this innovation is a special protective layer for the zinc anodes of the batteries. This layer addresses previous issues such as the growth of needle-like zinc structures—known as zinc dendrites—as well as unwanted chemical side reactions that trigger hydrogen formation and corrosion.
The research team, led by Prof. Roland A. Fischer, Chair of Inorganic and Metal-Organic Chemistry at the TUM School of Natural Sciences, uses a unique material for this purpose: a porous organic polymer called TpBD-2F. This material forms a stable, ultra-thin, and highly ordered film on the zinc anode, allowing zinc ions to flow efficiently through nano-channels while keeping water away from the anode.
Zinc Batteries as a Cost-Effective Alternative to Lithium-Ion Batteries
Da Lei, PhD student and lead author of the research published in Advanced Energy Materials, explains: "Zinc-ion batteries with this new protective layer could replace lithium-ion batteries in large-scale energy storage applications, such as in combination with solar or wind power plants. They last longer, are safer, and zinc is both cheaper and more readily available than lithium." While lithium remains the first choice for mobile applications like electric vehicles and portable devices, its higher costs and environmental impact make it less attractive for large-scale energy storage.
Prof. Roland A. Fischer adds: "This is truly a spectacular research result. We have shown that the chemical approach developed by Da Lei not only works but is also controllable. As fundamental researchers, we are primarily interested in new scientific principles—and here we have discovered one. We have already developed a first prototype in the form of a button cell. I see no reason why our findings couldn’t be translated to larger applications. Now, it's up to engineers to take up the idea and develop appropriate production processes."
Publication:
Da Lei, Roland A. Fischer et al.: Ion-Transport Kinetics and Interface Stability Augmentation of Zinc Anodes Based on Fluorinated Covalent Organic Framework Thin Films, published in Advanced Energy Materials on October 13, 2024, doi.org/10.1002/aenm.202403030
Additional Information:
Numerous TUM units participated in this interdisciplinary research project and international team—ranging from chemistry and physics to computer science, nanotechnology, and data science. Most of the TUM researchers involved are also part of the e-conversion Excellence Cluster, funded by the federal and state governments as part of the Excellence Initiative (https://www.e-conversion.de/en/).
Subject matter expert:
Prof. Dr. Roland A. Fischer
Technical University of Munich
Chair of Inorganic and Metal-Organic Chemistry
Tel: +49 89 289 - 13080
roland.fischer(at)tum.de
www.ch.nat.tum.de/amc
TUM Corporate Communications Center contact:
Ulrich Meyer
Press Spokesman
Tel: +49 89 289 22779
presse(at)tum.de
www.tum.de
Original article: https://www.tum.de/en/news-and-events/all-news/press-releases/details/significant-extension-of-zinc-battery-lifespan